By: OM1, September 2025
Authors: Chandrasekar Gopalakrishnan, Kathryn Starzyk, Luis Rangel, Eric Hanson, Michael Behling, Andrea Cohee, Beth Mitchell, Russel Burge. 1OM1, Inc., Boston, MA, USA; 2Eli Lilly and Company, Indianapolis, IN, USA; 3University of Cincinnati, Cincinnati, OH, USA
This blog is an extension of our award-winning ISPOR Annual 2025 poster: Predictors of Low Disease Activity/Remission Amongst Rheumatoid Arthritis Patients Experiencing Inadequate Response to Biologic/ Targeted Synthetic Disease-Modifying Antirheumatic Drugs
Rheumatoid Arthritis (RA) is a chronic autoimmune disorder characterized by persistent synovial inflammation, leading to bone and cartilage erosion and eventual joint destruction.1 Despite the availability of many advanced therapies on the market, numerous patients fail to achieve low disease activity (LDA) or remission, highlighting the need for more effective treatment strategies.
Our study identified that patients with comorbidities such as obesity, COPD, fibromyalgia, osteoporosis, and higher baseline Clinical Disease Activity Index (CDAI) scores were less likely to achieve remission.
Objective
🎯 This study aimed to identify predictors of treatment response in patients who previously experienced an inadequate response to a bDMARD or tsDMARD.
Methods
Study design: New-user retrospective cohort study
Data source: OM1 PremiOMTM RA (OM1, Boston, MA), a multisource real-world dataset with linked claims and EHR data on patients with RA in the USA
Study population: Adult patients with moderate-to-severe RA who initiated a b/tsDMARD between January 2013 to June 2024 and had used a different b/tsDMARD in the previous year.
- Patients had at least 365 days of baseline data available prior to and including the index date
- Patients had at least one CDAI score >10 in the 90 days prior to and including the index date (baseline), and at least one CDAI score in the 180 days following the index date (follow-up)
- Initiation of pre-specified b/tsDMARDs of interest: bDMARDs (abatacept, adalimumab, certolizumab, etanercept, golimumab, infliximab, rituximab, sarilumab, tocilizumab) and tsDMARDS (baricitinib, tofacitinib, upadacitinib)
Data Analysis:
- Primary outcome was LDA (CDAI < 10.0) or remission (CDAI < 2.8) measured at 6 months post treatment initiation
- Risk ratios and 95% CI were estimated using log-binomial regression to model the probability of achieving LDA/remission with baseline characteristics as predictors at 6- and 12-months follow-up
- Predictors included age, sex, race, ethnicity, insurance type, obesity, overweight AND weight-related comorbidity, fibromyalgia, depression, anxiety, COPD, cardiovascular disease, history of type 1 or 2 diabetes, RA disease duration, RF status, anti-CCP status, CRP level, baseline CDAI score, number of prior RA therapies used, prior use of GLP-1 antagonist, corticosteroids, bDMARDS, tsDMARDS, csDMARDS, csDMARDS, and whether the index treatment was a bDMARD vs. a tsDMARD.
- Estimated CDAI (eCDAI), a validated machine learning algorithm that estimates a patient’s CDAI score using clinical notes, 3 was used to supplement missing CDAI scores beyond 6 months of follow-up.
Results
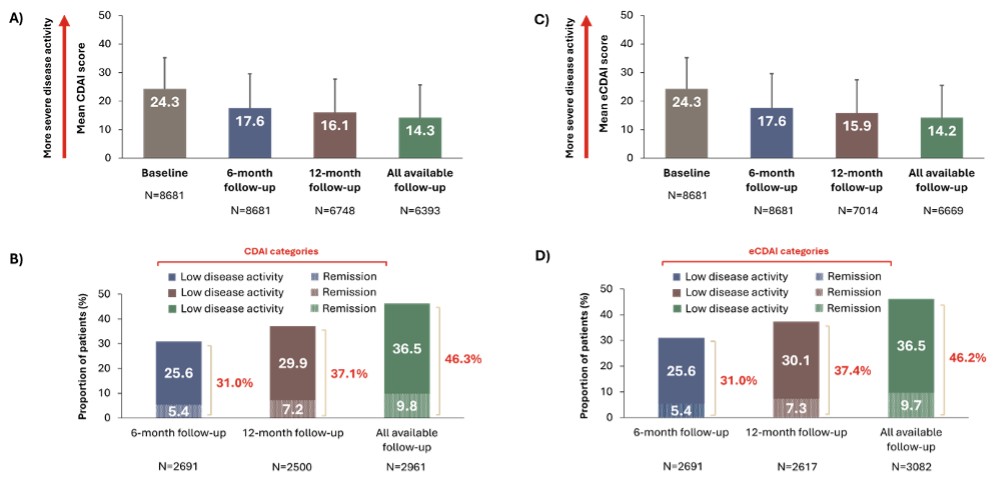
💡 At the 6-month follow-up, the mean CDAI decreased from 24.3 to 17.6, with 31.0% of patients achieving LDA/remission after 6 months of follow-up.
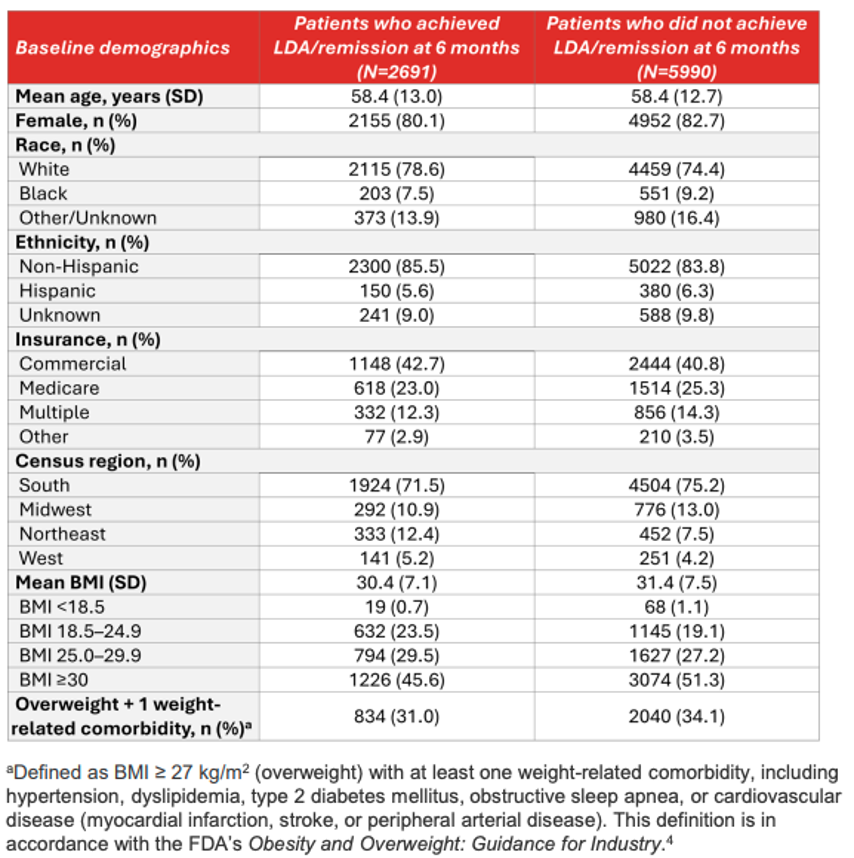
💡 Patients who achieved LDA/remission at 6 months were more likely to be White, have commercial insurance, reside in the Northeast, have a lower BMI, and less likely to be overweight or have a weight-related comorbidity.
💡Patients who achieved LDA/remission at 6 months were more likely to have moderate disease activity, have RA for ≥2 years, and less likely to have comorbidities, including fibromyalgia.
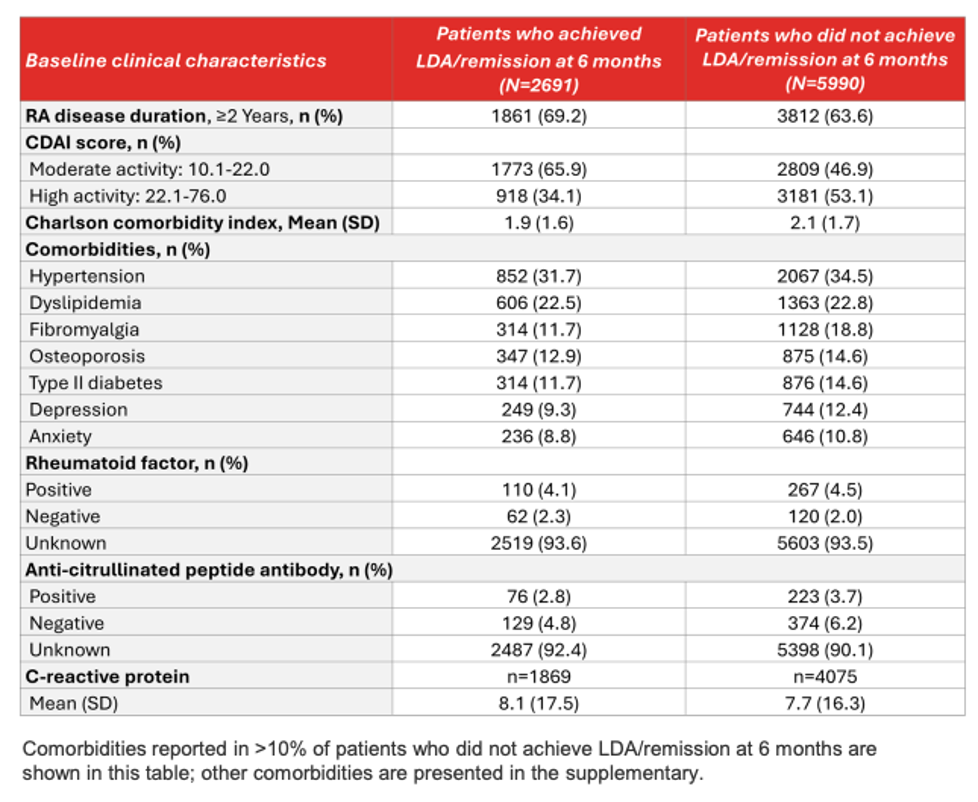
💡 Patients with obesity, osteoporosis, COPD, fibromyalgia, higher baseline CDAI scores, or those who initiated bDMARDs (vs. tsDMARDs) were less likely to achieve LDA/remission at 6 months.
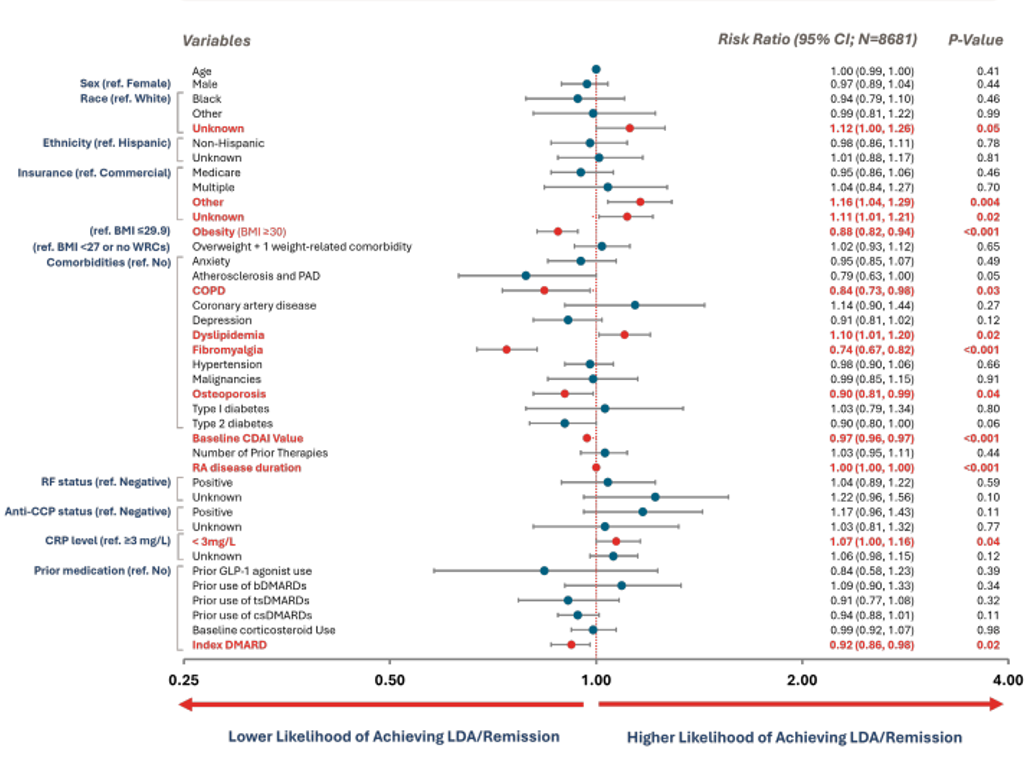
Multivariable associations of baseline characteristics on achieving LDA/remission at 6-month follow-up.
Statistical significance is denoted by values in bold red
C-Statistic=0.65 (A measure of the model’s ability to classify subjects achieving LDA at 6 months)
Note: The statistical significance observed for the ‘Unknown’ race category and CRP levels <3 mg/L may be influenced by missing data. Similarly, key laboratory variables such as RF status and anti-CCP status also had missing data. As a result, interpretation of findings related to these variables is limited and warrants caution.
Conclusion
- In this large real-world study of patients with moderate-to-severe RA and prior inadequate response to biologic/targeted synthetic DMARDs, over two-thirds (69%) and approximately two-thirds (≈63%) of those who started a new b/tsDMARDs failed to achieve low disease activity/remission at 6- and 12-month follow-up, respectively.
- Patients with obesity, comorbidities such as COPD, fibromyalgia, and osteoporosis, higher baseline CDAI scores, and those treated with bDMARDs (vs. tsDMARDs) at index were less likely to achieve LDA/remission after 6 months of follow-up. Similar results for obesity and other comorbidities were observed at the 12- month follow-up.
- These findings highlight the significant impact of obesity and comorbidities on the clinical management of RA, aligning with the ACR guidelines, which recommend maintaining a healthy weight to optimize long-term RA outcomes.5
- Future research should explore the interaction between comorbidities and RA disease activity to enable targeted interventions that improve outcomes.
Poster & Supplemental Materials
View the full poster and supplemental materials here.
References
- Jahid M, et al. Mediterr J Rheumatol. 2023;34(3):284–291.
- Deson S., Int. J. Clin. Rheumatol. 2024;19(6):208–210.
- Spencer AK, et al. RMD Open. 2021;7(3):e001781.
- FDA, 2025. Obesity and Overweight: Guidance for Industry. Available at: https://www.fda.gov/media/71252/download
- England BR, et al. Arthritis Rheumatol. 2023;1299–1311.
DISCLOSURES
Chandrasekar Gopalakrishnan, Kathryn Starzyk, and Luis Rangel: Employee of OM1, Inc.; Eric Hanson, Michael Behling, Andrea Cohee, Beth Mitchell, and Russel Burge: Employee and/or stockholder of Lilly.
ACKNOWLEDGMENT
Pranshu Roy, an employee of Eli Lilly Services India Pvt. Ltd, provided medical writing support.
