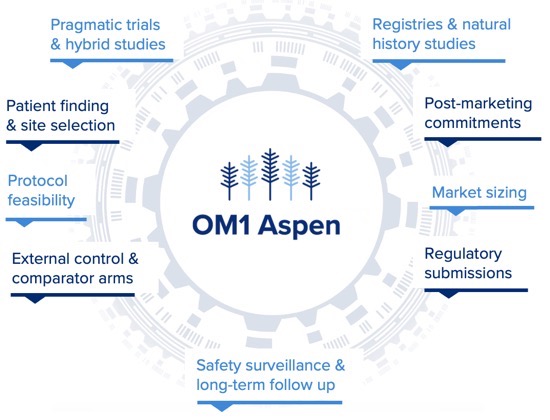
OM1 Aspen
Prospective and retrospective study automation platform and evidence generation solution
Request a meetingThought Leader in Registries
OM1 experts have been leading best practices in registries and observational studies for decades, including the AHRQ Registries for Evaluating Patient Outcomes: A User’s Guide, now in it’s 4th edition.
Changing the Research Paradigm
Study designs that use RWD offer a potential tool for the efficient generation of RWE to inform regulatory decision-making. Read: Using real-world evidence to support a changing paradigm for cancer screening: A commentary
Demonstrating Effectiveness
OM1 Aspen supports large-scale comparative effectiveness and outcomes studies. Read about our largest study: Mammographic Screening in Routine Practice: Multisite Study of Digital Breast Tomosynthesis and Digital Mammography Screenings
Ready to talk?
See how OM1 Aspen can help you reach your research goals faster and more efficiently.